The Regulations: 211.25 Personnel Qualifications

“Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, to enable that person to perform the assigned functions.” … “Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency to assure that employees remain familiar with CGMP requirements applicable to them.”* In other words, you need Annual Refresher CGMP Training!
Topics offered by SkillsPlus International Inc. can be customized, therefore, to better meet your training needs. Here are some customizing features:

- you select the standard presentation or specific subtopics within each of the Subparts addressed within the New Generation Series, in other words, you customize the content!
- select the start date and the end date for training access, therefore, students schedule training at their convenience.
- you set the passing grade requirement, so you know your employees have learned required information.
- determine who, in addition to the student, will automatically receive a copy of the completion certificate for each student, so tracking your training documentation is easier.
- if you need additional customizing for very unique plant site needs, let’s discuss what needs customizing and we can present to you a tailored quotation.
Email us or give us a call to discuss your training needs. Simply contact us!
CGMP Training: A New Generation
for annual refresher CGMP training
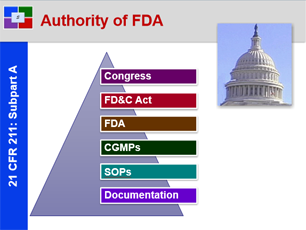
- 21 CFR 211: Pure, Safe and Effective – Overview of 21 CFR 211
- Your Personal Responsibilities: Organization and Personnel – Detailed review of 21 CFR 211 – Subpart B
- Foundation to Facility – Detailed review of 21 CFR 211 – Subpart C
- Maintaining Compliant Equipment Practices – Detailed review of 21 CFR 211 – Subpart D
- Supplier to Plant – Detailed review of 21 CFR 211 – Subpart E
- Plan To Production – Detailed review of 21 CFR 211 – Subpart F
- Plant To Patient – Detailed review of 21 CFR 211 – Subpart G, H, & K
- Laboratory To Release – Detailed review of 21 CFR 211 – Subpart I
- Proper Documentation Practices – Detailed review of 21 CFR 211 – Subpart J
- 21 CFR Part 820: Overview of QSR – Detailed review of 21 CFR 820 – Subpart A-O
Explore each topic above in more detail. Get ready for Annual Refresher CGMP Training!
Email us or give us a call to discuss your training needs. Simply contact us!
