Course Descriptions
Select from these to create a custom course for your CGMP Training: A New Generation.

- 21 CFR 211: Pure, Safe and Effective – Overview of 21 CFR 211
- Your Personal Responsibilities: Organization and Personnel – Detailed review of 21 CFR 211 – Subpart B
- Foundation to Facility – Detailed review of 21 CFR 211 – Subpart C
- Maintaining Compliant Equipment Practices – Detailed review of 21 CFR 211 – Subpart D
- Supplier to Plant – Detailed review of 21 CFR 211 – Subpart E
- Plan To Production – Detailed review of 21 CFR 211 – Subpart F
- Plant To Patient – Detailed review of 21 CFR 211 – Subpart G, H, & K
- Laboratory To Release – Detailed review of 21 CFR 211 – Subpart I
- Proper Documentation Practices – Detailed review of 21 CFR 211 – Subpart J
- 21 CFR Part 820: Overview of QSR – Detailed review of 21 CFR 820 – Subpart A-O
Course Descriptions For CGMP Training: A New Generation Series
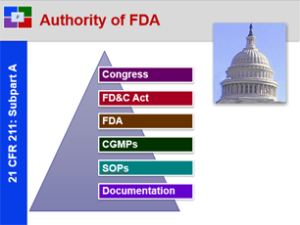
21 CFR 211: Pure, Safe and Effective – Overview of 21 CFR 211
This course provides a solid basic understanding of the CGMP Regulations for Pharmaceutical Manufacturers and Packagers. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers.
Email us or give us a call to discuss your training needs. Simply contact us for more information about CGMP Training: A New Generation!
All of the courses below can be customized by selecting or excluding specific topics:

Your Personal Responsibilities: Organization and Personnel – 21 CFR 211 – Subpart B
This course provides a detailed explanation into: the role of the Quality Unit; what is really meant by “training, education, and experience”; the application of the adequate staffing requirements; source of contamination; the history of handwashing; hand washing methods in a world with Covid-19; basic gowning; and reporting of illness and lesions. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. Consider this topic for handwashing refresher training also.
Email us or give us a call to discuss your training needs. Simply contact us for more information about CGMP Training: A New Generation!
Foundation to Facility – 21 CFR 211 – Subpart C
This course is offered in two levels. A basic level for all plant employees and an advanced level for employees responsible for the design and construction or renovation of production and laboratory facilities. This complete course provides a detailed discussion of: the design and use of space; the requirements for adequate construction; the uses of defined spaces; a comprehensive survey of plant utilities; a concise discussion of air filtration; an in-depth discussion of temperature and humidity; an explanation of air classifications; the requirements for plumbing systems, the standards for water storage; a discussion of steam and chilled water; pest control requirements with insights from pest control technicians; facility cleaning and maintenance requirements, and a review of environmental monitoring requirements. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great orientation for engineers new to pharmaceuticals and specifically the CGMP. It is also a good review for engineers as an annual refresher or prior to launching new facility based projects.
Email us or give us a call to discuss your training needs. Simply contact us!

Maintaining Compliant Equipment Practices – 21 CFR 211 – Subpart D
This course provides a detailed explanation into: equipment design requirements; the concerns and requirement of equipment materials of construction; the various levels of equipment cleaning; the requirements for equipment maintenance and calibration; equipment cleaning and maintenance documentation; filters are discussed as equipment; the role of procedures and the requirements for change control, qualification and validation; the gold standard for training; and a detailed examination of process qualification and validation. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great review for the validation team prior to the design or execution of a validation protocol.
Email us or give us a call to discuss your training needs. Simply contact us!
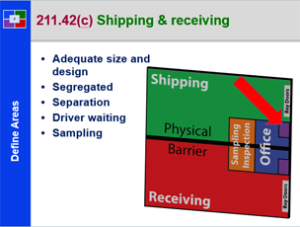
Supplier to Plant – 21 CFR 211 – Subpart E
This course provides a detailed explanation into a components, drug product containers and closures, specifically: a discussion of receipt of materials; a detailed discussion of random and representative sampling; a review of the requirements to sample, test and inspect materials; the proper use of materials in the plant; a review of USP storage containers for products; the need for examination and usage activities and documentation; and a review of component records; This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great review for employees new to materials management and supply chain.
Email us or give us a call to discuss your training needs. Simply contact us!
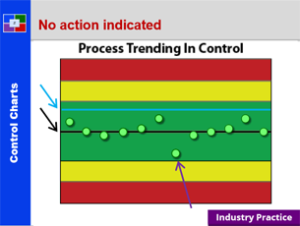
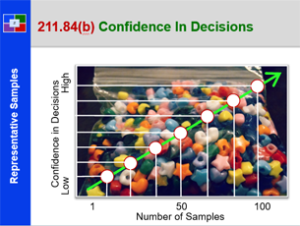
Plan To Production – 21 CFR 211 – Subpart F
This course provides a detailed explanation into production and process controls, specifically: a summary review orients the student to the many requirements; a detailed discussion of sampling; a thorough explanation of control charts; an in depth introduction to validation; a detailed review of validation regulations; an explanation of validation strategies; an explanation of the qualification of challenge within validation; and what validation means to everyone. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great review for employees new to validation teams, change control teams, and the quality unit.
Email us or give us a call to discuss your training needs. Simply contact us!

Plant To Patient- 21 CFR 211 – Subpart G, H, & K
This course provides a detailed explanation into three short subparts of the regulations, specifically, Packaging and Labeling Control, Holding and Distribution, and Returned and Salvaged Drug Products: a review of label issuance controls; a summary of packaging and labeling operation regulations; a detailed explanation of expiration dates; a review of the tamper-evident requirements; warehouse and distribution regulations; the requirement for returned and salvaged drug products; handling of complaints; and the basic requirements for recalls. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great review for employees new to validation teams, change control teams, and the quality unit. Email us or give us a call to discuss your training needs. Simply contact us!
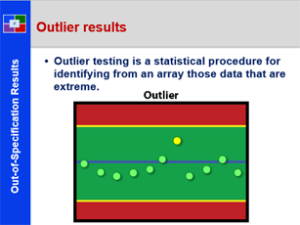
Laboratory to Release- 21 CFR 211 – Subpart I
This course provides a detailed explanation laboratory regulations and practices, specifically: a review of laboratory control general expectations; specific attention is given to unique laboratory documentation practices; and explanation of specifications and acceptance criteria; a detailed discussion of the components of method validation; a historical and current review for handling out-of-specification test results; an in depth discussion of stability study concepts and practices; and the collection, storage and use of reverse samples. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great review for employees new to the pharmaceutical laboratory.
Email us or give us a call to discuss your training needs. Simply contact us!

Proper Documentation Practices – 21 CFR 211 – Subpart J
This course provides a detailed explanation of documentation regulations and industry common practices for paper-based and electronic records, specifically: a review of the CGMP regulation requirements; proper data entry practices; proper error correction practices; unacceptable data entry practices; unacceptable error correction practices; and electronic record system (21CFR11) design, use, security and backup requirements. This is a great starting course for all all employees from pharmaceutical, biologic and bio-tech drug manufacturers. It is also a great review for employees migrating a firm from a paper-based record environment to the electronic record world.
Email us or give us a call to discuss your training needs. Simply contact us!
Contact us for more information about CGMP Training: A New Generation.
Request a CGMP training quote if you’re ready to act on CGMP Training: A New Generation.
